Gamp 5 Online Training
This highly interactive course describes how the GAMP Good Practice Guide. Gamp 5 training.

Image Result For Process Flow Diagram For Antibody Development Looks Like Process Flow Diagram Process Control Flow Diagram
GAMP 5 21 CFR Part 11 Compliance with Computer System Validation 02nd - 03rd June Mumbai 06th - 07th June Chandigarh 13th - 14th June Hyderabad since.

Gamp 5 online training. Request a quote for an onsite or online virtual course for an agreed number of delegates from your site. While theres nothing that requires this structure or these titles its helpful to see the breadth of involvement in validation. The basis of the Live Online Training will be the current requirements for the validation of computerised systems like GAMP and their GxP-oriented application in practice.
- The GAMP 5 Approach Live Online Training on 17 November and 18-20 November 2020 23 March 2021 and 24-26 March 2021 Including implications of EU GMP Annex 11 computerised systems GMP Certification Programme Certified Computer Validation Manager. The course covers recommended good practice based on a life cycle approach for the development. Experts from the pharmaceutical industry and from the GAMP Committee will show you efficient ways to.
The course content can be tailored to meet your requirements. The course content can be tailored to meet your requirements. This computerised systems validation training course covers the essential principles on how to use a risk-based approach in Computerised Systems Validation CSV.
GAMP Computerised Systems Validation Training Virtual This training course on how to validate computerised systems covers the essential principles on how to use a risk-based approach in Computer Systems Validation CSV. This fundamental course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and explores tried tested. The GAMP 5 Training Course can be delivered in a hotel one of our offices your premises or online via a virtual classroom.
GAMP 5 defines a set of fairly typical roles in validation. The approach matured in the 2005 ISPE GAMP Good Practice Guide. Whilst course started out being heavily focused as a GAMP 5 Training Course the scope and application has been broadened.
Takes place on 2 nd 4 th June 2020. A Risk Based Approach to GxP Process Control Systems may be applied to achieve process control systems that are fit for intended use and meet current regulatory requirements. True ignorance is not the absense of knowledge but the refusal to aquire it.
The course provides a broad overview of GMP requirements for computerised systems and is designed for professionals seeking training on a practical. GAMP 5 GxP Process Control Training Course. This course offers a high level compliance review of the.
A Risk-Based Approach to Compliant Electronic Records and Signatures with incorporation of aspects of ISO 14971 Medical Devices Appli-. The trainings and workshops can be given worldwide in various languages including Dutch German English and Polish. Request a quote for an onsite or online virtual course for an agreed number of delegates from your site.
1980 training the professionals R Two days training on. The main roles and responsibilities include. The development of the GAMP 5 risk man-agement approach has its antecedents in the FMEA-based risk assessment tool published in GAMP 4 in 2001.
GAMP 5 provides pragmatic and practical industry guidance to achieve compliant computerized systems fit for intended use in an efficient and effective manner. This technical document describes a flexible risk-based approach to compliant GxP regulated computerized systems based on scalable specification and verification. Rescop regularly provides in-house training and workshops for employees and inspectors within the pharmaceutical and medical device industry.
A big advantage of the in-house training is that we can focus on. Understanding Validation GAMP 5 21 CFR Part 11 and Data Integrity Training Programme. The GAMP 5 3 Day Training Course can be delivered in a hotel one of our offices your premises or online via a virtual classroom.
The Understanding Validation GAMP 5 21 CFR Part 11 and Data Integrity 3 Day Training Programme provides learners with the opportunity to attend one course alone a mix of the following courses or all three in their entirety over three consecutive days.

Gamp Training Courses Ispe International Society For Pharmaceutical Engineering

Site Master File Example 4 Site Master Master Site
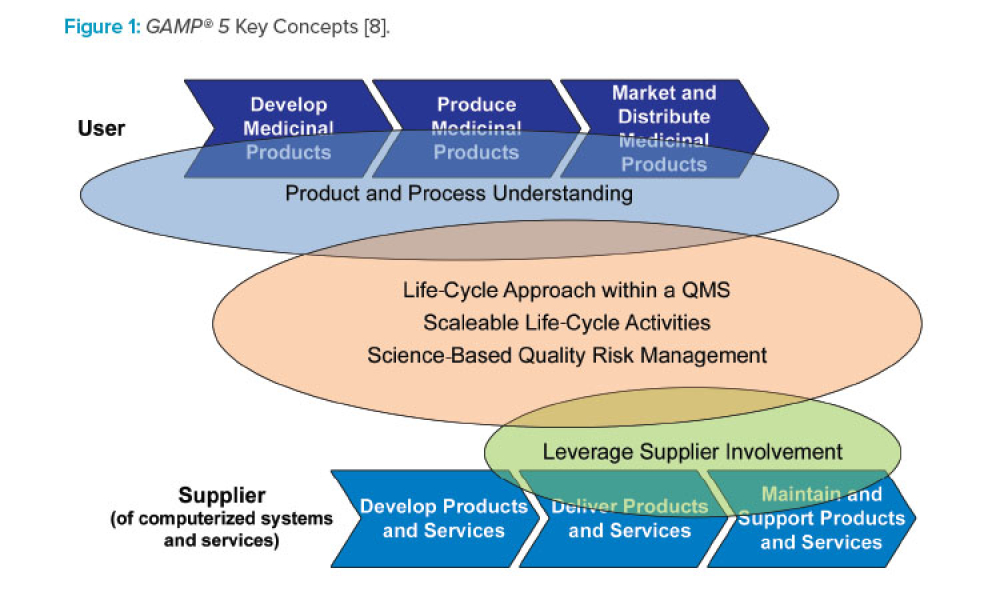
Gamp Resources Ispe International Society For Pharmaceutical Engineering

Regulatory Science Professionals Are In Demand A Career In Regulatory Affairs Can Take Many Paths E G Clinic Science Programs Regulatory Affairs Regulatory

Codebeamer X Gamp 5 Template Introduction Intland Software
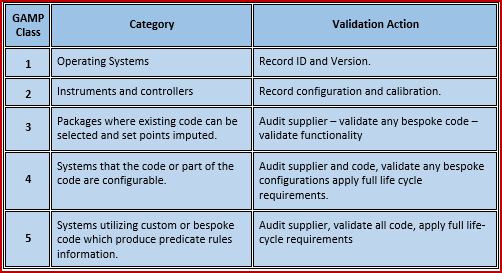
Gamp 5 Fda Mhra Eu Who Cgmp Flcv Sop S Sop S Gxp

Gamp 5 Guide Compliant Gxp Computerized Systems Ispe International Society For Pharmaceutical Engineering

Key Principles Of Gamp 5 For Computer System Validation Youtube

Computer Validation The Gamp 5 Approach Eca Academy

Intland Software Announces Pharma Gamp 5 Validation Quality Risk Management Template Intland Software

Gamp 5 In Pharmaceuticals Pharmaceutical Pharmaceutical Manufacturing Change Control

Regulatory Affairs Organization For Professionals In Medical Devices Pharmaceutical Biologic And Ivd Industr Regulatory Affairs Career Advancement Regulatory

Summer Camping Badges Badges Logo Badge Summer

Live Online Training Computerised System Validation The Gamp 5 Approach Eca Academy





Post a Comment for "Gamp 5 Online Training"
Post a Comment